Harrow and Hillingdon Geological Society
Isotopes
Home | Monthly Meetings | Field Trips | Exhibitions | Other Activities | Members Pages | Useful Links
This write up was kindly provided by Dr George Darling.
Isotopes and Earth Systems: Cosmic Connections
The term ‘earth systems’ is used here to denote systems operating on the planet-wide scale. The ‘cosmic connections’ of the title refers to earth systems which are, or have been, affected in some way by Earth’s position in the solar system. However, first a few words of introduction: isotopes of a given element have the same chemical properties but differ slightly in physical properties such as density. They may be stable (i.e. permanent) or radioactive (i.e. subject to decay). The larger the discrepancy between the numbers of protons and neutrons, the more likely an atom is to be radioactive. In general stable isotopes are used as indicators of processes (e.g. evaporation of water), while the radio-isotopes are used as indicators of elapsed time (e.g. potassium-argon dating). This talk covers three earth systems of which the presenter has had some experience: helium isotopes, radiocarbon dating, and the stable isotopes of the water molecule.
Helium has two stable isotopes: 3He and 4He, the latter very much in the majority. The 3He/4He ratios in both meteorites and the solar wind are about 230 times higher than the present ratio of the earth’s atmosphere. It is assumed that at the birth of the solar system around 4.5 billion years ago, the earth had a similar bulk 3He/4He ratio. Owing to the production of 4He from the uranium-series decay chain, such high ratios are not likely to be encountered today. However, 3He/4He ratios of up to 50 times atmospheric are still found in areas of flood basalt volcanism believed by many researchers to be due to mantle plumes. This has given rise to the concept of the ‘layered mantle’, with an upper layer well-mixed by plate tectonic processes, and a lower layer with a much more ‘primitive’ composition (for one consequence of this see Figure 1). On a more local scale, helium isotope ratios can be used for practical purposes such as geothermal prospecting or surface-exposure dating. This latter geomorphological application relies on 3He being produced via the cosmic ray bombardment of mineral surfaces (a so-called spallation process). Away from the earth, cosmic ray bombardment of the moon is considered by some to mean that the surface layers must be comparatively rich in 3He. It has been proposed that this be mined as a source of fuel for the fusion reactors that may one day be providing us with electrical power.
All living plants and animals absorb 14C produced in the atmosphere by cosmic ray spallation of 14N. Radiocarbon dating relies fundamentally on the radioactive decay of 14C that starts when the organism dies. Since the half-life of 14C is known, in theory the age of the organism (or rather, the time since its death) can be worked out using the simple decay equation, up to a limit of 40–50 thousand years. However, the flux of cosmic rays has varied over that time mainly owing to changes in the Sun’s output. This means that radiocarbon ages need to be ‘calibrated’ if they are going to provide archaeologically useful dates. This can be done back to about 5000 years by calibration against ‘proxies’ like the rings of long-lived trees such as bristlecone pines, or of long-dead but still well-preserved trees such as ‘bog oaks’. Beyond this, calibrations have been made against proxies such as lake sediment carbonate (see Figure 2 for an example).
The stable oxygen and hydrogen isotope ratios of water can provide important information on processes occurring in the hydrological cycle, both now and in the past. When waters evaporate from the tropical oceans, the heavier isotopes are more reluctant to leave the ocean, meaning that air mass moisture is ‘depleted’ in the heavy isotopes. When rain subsequently falls from the air mass, the heavy isotopes that did make it into the atmosphere tend to fall out earlier. The net result of this is that rainfall becomes more depleted as air temperature drops. For this reason, a world map of rainfall isotopes shows increasingly negative compositions towards high-altitude and high-latitude regions. This is because the ocean is assigned a arbitrary delta (δ) value of 0 ‰ (‘permil’) for both oxygen and hydrogen isotope ratios. In the past, however, when very large amounts of water were ‘locked up’ in icecaps owing to glaciation, the oceanic isotope composition became greater than 0 ‰ (i.e. it went positive) because the hydrological cycle was disrupted. Since seawater imparts its oxygen isotope composition to the foraminifera living in it, and those foraminifera can be radiocarbon-dated, changes in oceanic δ18O during the ice age have been worked out in some detail. The chronology largely supports the contention of the early-twentieth-century geophysicist Milutin Milankovich, that changes in the precession and obliquity of the earth’s axis, combined with changes in orbital eccentricity, are responsible for the observed pattern of glaciations during the last ice age (see Figure 3) and, indeed, during previous ones.
It is believed from evidence largely based on isotope ratios of the noble gases (of which helium is a member along with neon, argon, krypton and xenon) that the earth’s atmosphere has effectively been closed since about 200 million years after solar system condensation, i.e. has remained stable in its water content for the best part of 4.5 billion years, and that O and H isotope ratios in the hydrosphere remain similar to the original endowment. Compared with Earth, Mars and especially Venus are very enriched in 18O and 2H due to the stripping away of their store of water. In the case of Venus this is promoted by very high surface temperatures, while for Mars it is due to its low gravity compared to that of Earth.
In conclusion, this talk has considered only three of the many fields in which isotopes are used in the earth sciences. Indeed, it is probable that no branch of earth sciences remains untouched by isotopic methods. Isotopes – and unfortunately the myriad different ways in which their units are expressed – are here to stay!
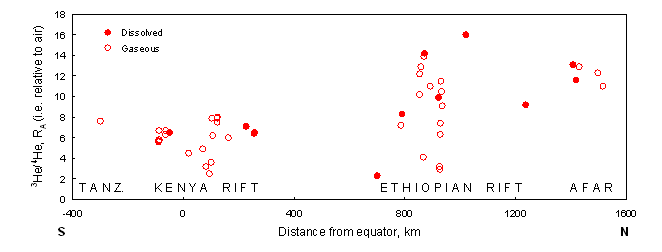
Figure 1
Significantly higher peak 3He/4He ratios in thermal waters and gases from the Main Ethiopian Rift and the Afar Triangle compared to those from Kenya and Tanzania are believed to reflect differing causes of updoming: a plume from the lower mantle for the former, and much shallower upper mantle convection for the latter. Based on data of the British Geological Survey.
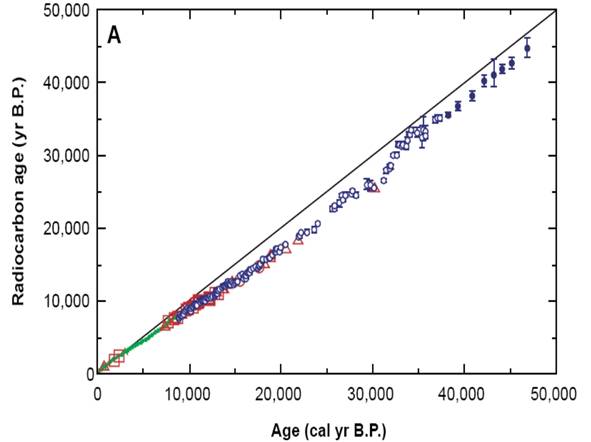
Figure 2
Example of a radiocarbon calibration curve based on measuring the 14C activities of individual lake varves (from Kitigawa & van der Plicht, Science 279: 1187–1190, 1998). Discrepancies between this curve and the simple 14C decay age line can be up to around 5000 years.
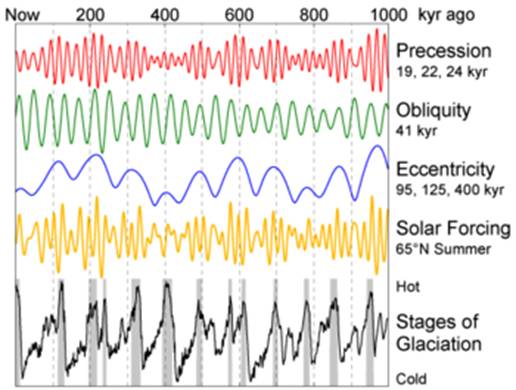
Figure 3
Diagram summarising the Milankovitch theory of the causes of glaciation on Earth, based on the effects of axial precession and obliquity combined with orbital eccentricity. From http://en.wikipedia.org/wiki/Milankovitch_cycles.